品质至上,客户至上,您的满意就是我们的目标
当前位置: 首页 > 新闻动态
科学家利用Videometer多光谱成像系统发表基于自荧光成像的大豆种子成熟期识别方法
发表时间: 点击:797
来源:北京博普特科技有限公司
分享:
最近,来自巴西的科学家利用Videometer多光谱成像系统在知名期刊“Front. Plant Sci.,”上发表了题为“A Reliable Method to Recognize Soybean Seed Maturation Stages Based on Autofluorescence-Spectral Imaging Combined With Machine Learning Algorithms”的文章,这是利用该系统在自荧光成像领域发表的数篇文章之一。
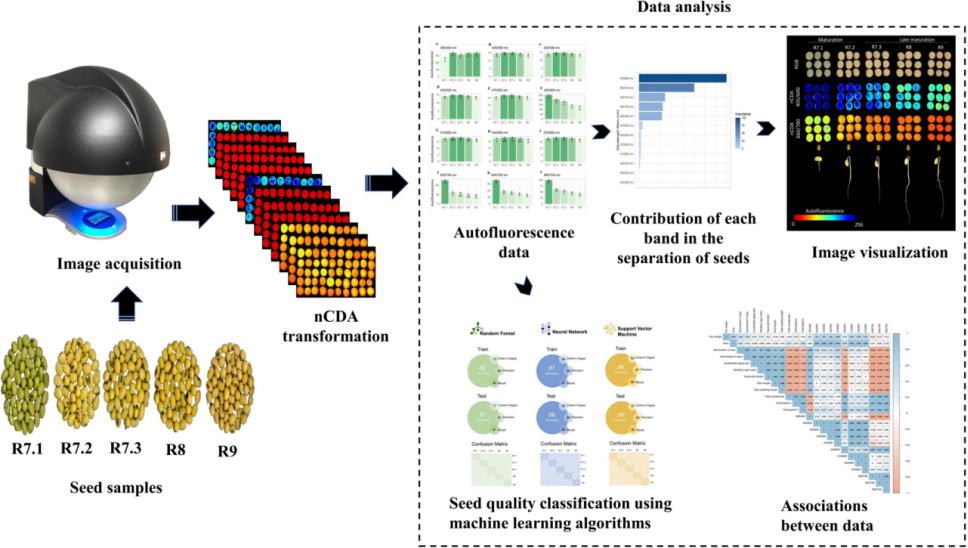
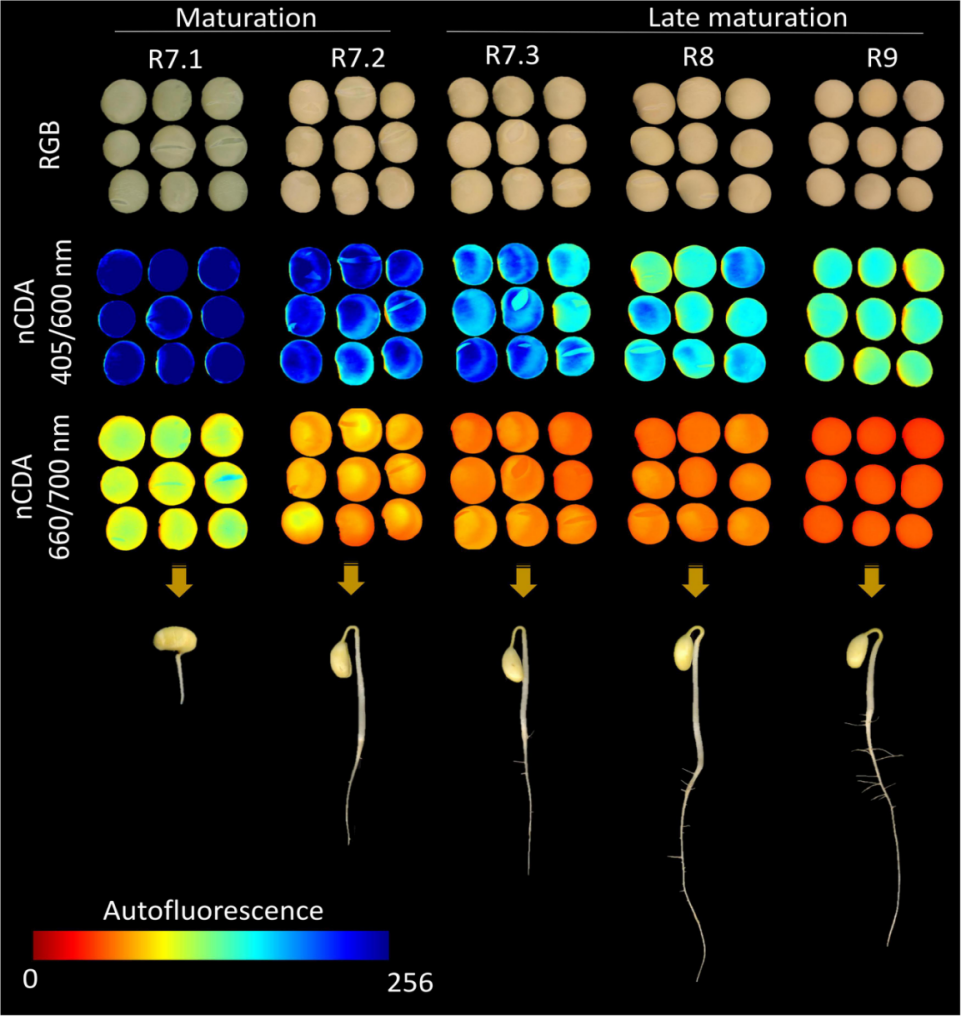
近年来,技术创新在种子质量诊断方面取得了重大进展。具有优异生理质量的种子是那些具有最高生理成熟度的种子,而快速和精确的分离方法的结合有助于提高田间表现。自体荧光光谱成像是一种基于种子组织中荧光团荧光信号的创新技术,对种子质量具有生物学意义。因此,通过这种技术,可以对不同成熟阶段的种子进行分类。为了测试这一点,我们生产了一种商业品种(MG/BR 46“Conquista”)的植物,并在五个繁殖(R)阶段收集种子:R7.1(成熟开始)、R7.2(大量成熟)、R7.3(种子与母株分离)、R8(收获点)和R9(最终成熟)。从在不同激发/发射组合下捕获的图像中提取自体荧光信号。同时,我们研究了不同成熟期种子的物理参数、发芽、活力和色素的动态。为了验证基于自体荧光光谱成像预测种子成熟阶段的准确性,我们基于三种算法创建了机器学习模型:(i)随机森林、(ii)神经网络和(iii)支持向量机。在这里,我们报道了前所未有地使用自体荧光光谱技术来分类大豆种子的成熟阶段,特别是使用叶绿素a(660/700nm)和b(405/600nm)的激发/发射组合。总之,机器学习算法在种子成熟的不同阶段表现出高性能分割。结果表明,大豆种子的成熟阶段在叶绿素的波长上具有自身的荧光光谱特性,这允许将该技术用作种子成熟度和优异生理质量的标记。
自荧光光谱成像和数据提取
使用VideometerLab4从25个种子的四个重复中捕获多光谱图像™ 仪器(Videometer A/S,Herlev,丹麦)。该系统可以使用不同激发波长的LED发光二极管结合光学滤波器(长通滤波器)捕获高分辨率自体荧光光谱图像(2192×2192像素)。使用双面胶带将种子放置在乙酸片(5.0cm×8.5cm)上的相同位置(左侧的种脐)。在图像采集之前,调整光设置以优化每种照明类型的选通时间,从而获得更好的信噪比,以直接比较捕获的图像。使用代表性样本校准灯光设置,并保存所有后续图像。使用不同的激发/发射组合在1分钟内以一个序列生成每个样品的自荧光光谱图像:365/400、405/500、430/500、450/500、470/500、405/600、515/600、540/600、570/600、630/700、645/700和660/700 nm。使用VideometerLab提取自体荧光数据™ 软件(3.14.9版)。为此,应用基于阈值的分割图像技术将种子从背景中分离出来,背景由零表示。在分割的种子中,应用标准化正则判别分析(nCDA)算法来逐像素突出每个激发/发射组合的自体荧光信号。nCDA算法使用10%的修剪平均值,消除了离群值(数据中最低的10%和最高的10%)的影响(Barboza da Silva等人,2021b),将灰度图像转换为带有红-绿-蓝色码的分数图像(Bianchini等人,2021)。自体荧光图像中的像素值取决于荧光团的浓度。也使用相同的传感器采集RGB图像。他们被捕获以将肉眼不可见的种子特征与自体荧光光谱图像进行比较。
A Reliable Method to Recognize Soybean Seed Maturation Stages Based on Autofluorescence-Spectral Imaging Combined With Machine Learning Algorithms
Front. Plant Sci., 14 June 2022
Sec. Technical Advances in Plant Science
https://doi.org/10.3389/fpls.2022.914287
In recent years, technological innovations have allowed significant advances in the diagnosis of seed quality. Seeds with superior physiological quality are those with the highest level of physiological maturity and the integration of rapid and precise methods to separate them contributes to better performance in the field. Autofluorescence-spectral imaging is an innovative technique based on fluorescence signals from fluorophores present in seed tissues, which have biological implications for seed quality. Thus, through this technique, it would be possible to classify seeds in different maturation stages. To test this, we produced plants of a commercial cultivar (MG/BR 46 “Conquista”) and collected the seeds at five reproductive (R) stages: R7.1 (beginning of maturity), R7.2 (mass maturity), R7.3 (seed disconnected from the mother plant), R8 (harvest point), and R9 (final maturity). Autofluorescence signals were extracted from images captured at different excitation/emission combinations. In parallel, we investigated physical parameters, germination, vigor and the dynamics of pigments in seeds from different maturation stages. To verify the accuracy in predicting the seed maturation stages based on autofluorescence-spectral imaging, we created machine learning models based on three algorithms: (i) random forest, (ii) neural network, and (iii) support vector machine. Here, we reported the unprecedented use of the autofluorescence-spectral technique to classify the maturation stages of soybean seeds, especially using the excitation/emission combination of chlorophyll a (660/700 nm) and b (405/600 nm). Taken together, the machine learning algorithms showed high performance segmenting the different stages of seed maturation. In summary, our results demonstrated that the maturation stages of soybean seeds have their autofluorescence-spectral identity in the wavelengths of chlorophylls, which allows the use of this technique as a marker of seed maturity and superior physiological quality.
Autofluorescence-Spectral Imaging and Data Extraction
Multispectral images were captured from four replicates of 25 seeds using a VideometerLab4™ instrument (Videometer A/S, Herlev, Denmark). This system can capture high resolution autofluorescence-spectral images (2192 × 2192 pixels) using light-emitting diodes at different excitation wavelengths combined with optical filters (long-pass filters). The seeds were placed on an acetate sheet (5.0 cm × 8.5 cm) in the same position (hilum to the left) using double-sided adhesive tape. Before image acquisition, the light setting was adjusted to optimize the strobe time of each illumination type, resulting in a better signal-to-noise ratio such that the captured images could be directly comparable. Light setup was calibrated using a representative sample and saved for all subsequent images. Autofluorescence-spectral images of each sample were generated in one sequence during 1 min using different excitation/emission combinations: 365/400, 405/500, 430/500, 450/500, 470/500, 405/600, 515/600, 540/600, 570/600, 630/700, 645/700, and 660/700 nm. Autofluorescence data were extracted using VideometerLab™ software (version 3.14.9). For this, a segmentation image technique based on thresholding was applied to separate the seeds from the background, which was represented by zero. In the segmented seeds, a normalized canonical discriminant analysis (nCDA) algorithm was applied to highlight the autofluorescence signals pixel-to-pixel for each excitation/emission combination. The nCDA algorithm uses a 10% trimmed mean, eliminating the influence of outliers (the lowest 10% and the highest 10% of the data) (Barboza da Silva et al., 2021b), transforming grayscale images into score images with red-green-blue color codes (Bianchini et al., 2021). The pixel values in the autofluorescence images depend on the concentration of fluorophores. RGB images were also acquired using the same sensor. They were captured to compare seed characteristics that are not visible to the naked eye with autofluorescence-spectral images.